
FIREHAWK®
Firehawk® Rapamycin Target Eluting Coronary Stent System Features

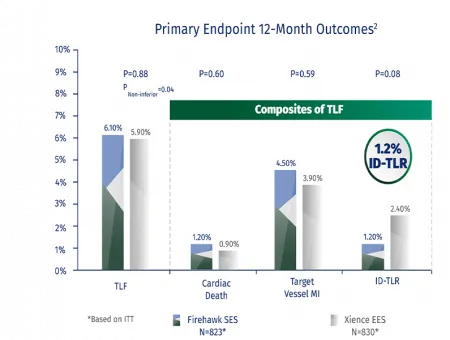
Clinical Evidence
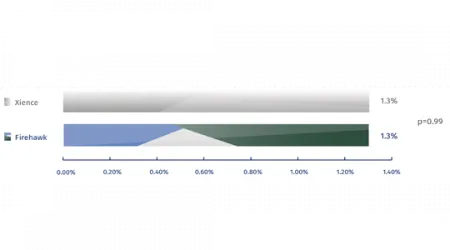
Prob/Definite stent Thrombosis at 1 year
Product Specification
Stent Material: | L605 CoCr |
Polymer: | PLA, 100% Biodegradable |
Drug: | Rapamycin (Sirolimus) |
Drug Dosage: | 0.3 µg/mm2 |
Strut Thickness: | 86µm (Ø2.25mm - 3.0mm) |
Nomial Pressure: | 10 atm |
Rated Burst Pressure: | 16 atm (Ø2.25mm - 3.5mm) |
Mean Recoil: | 3.4% |
Mean Foreshortening: | 1.95% - 5.30% |
| Length /Ø | 13mm | 16mm | 18mm | 21mm | 23mm | 26mm | 29mm | 31mm | 33mm | 35mm | 38mm |
| 2.25mm | RV2213 | RV2216 | RV2218 | RV2221 | RV2223 | RV2226 | RV2229 | - | - | - | - |
| 2.5mm | RV2513 | RV2516 | RV2518 | RV2521 | RV2523 | RV2526 | RV2529 | RV2531 | RV2533 | - | - |
| 2.75mm | RV2713 | RV2716 | RV2718 | RV2721 | RV2723 | RV2726 | RV2729 | RV2731 | RV2733 | RV2735 | RV2738 |
| 3.0mm | RV3013 | RV3016 | RV3018 | RV3021 | RV3023 | RV3026 | RV3029 | RV3031 | RV3033 | RV3035 | RV3038 |
| 3.5mm | RV3513 | RV3516 | RV3518 | RV3521 | RV3523 | RV3526 | RV3529 | RV3531 | RV3533 | RV3535 | RV3538 |
| 4.0mm | RV4013 | RV4016 | RV4018 | RV4021 | RV4023 | RV4026 | RV4029 | RV4031 | RV4033 | RV4035 | RV4038 |
1 Lansky, A., Wijns, W., Xu, B.,…Baumbach, A.(2018). Targeted therapy with a localised abluminal groove, low-dose sirolimus-eluting, biodegradable polymer coronary stent (TARGET All Comers): a multicentre, open-label, randomised non-inferiority trial. Lancet. Doi: 10.1016/S0140-6736(18)31649-0